Archive: 2025/12

Grapefruit Juice Interactions: Why Some Medications Are Affected
Grapefruit juice can dangerously increase levels of certain medications by blocking enzymes that break them down. Over 85 drugs, including statins and blood pressure meds, are affected. Avoid grapefruit entirely if you're on one of them.
How to Handle Missed Pediatric Medication Doses Safely: Step-by-Step Guide for Parents
Learn how to safely handle missed pediatric medication doses without doubling up. Step-by-step guidance based on clinical guidelines for once-daily, twice-daily, and other dosing schedules. Prevent errors and keep your child safe.

Topical Medication Allergies: How Contact Dermatitis Develops and How to Treat It
Topical medication allergies cause contact dermatitis in 10-17% of patch-tested patients. Learn how to spot the signs, which drugs trigger reactions, and how patch testing can finally give you answers.
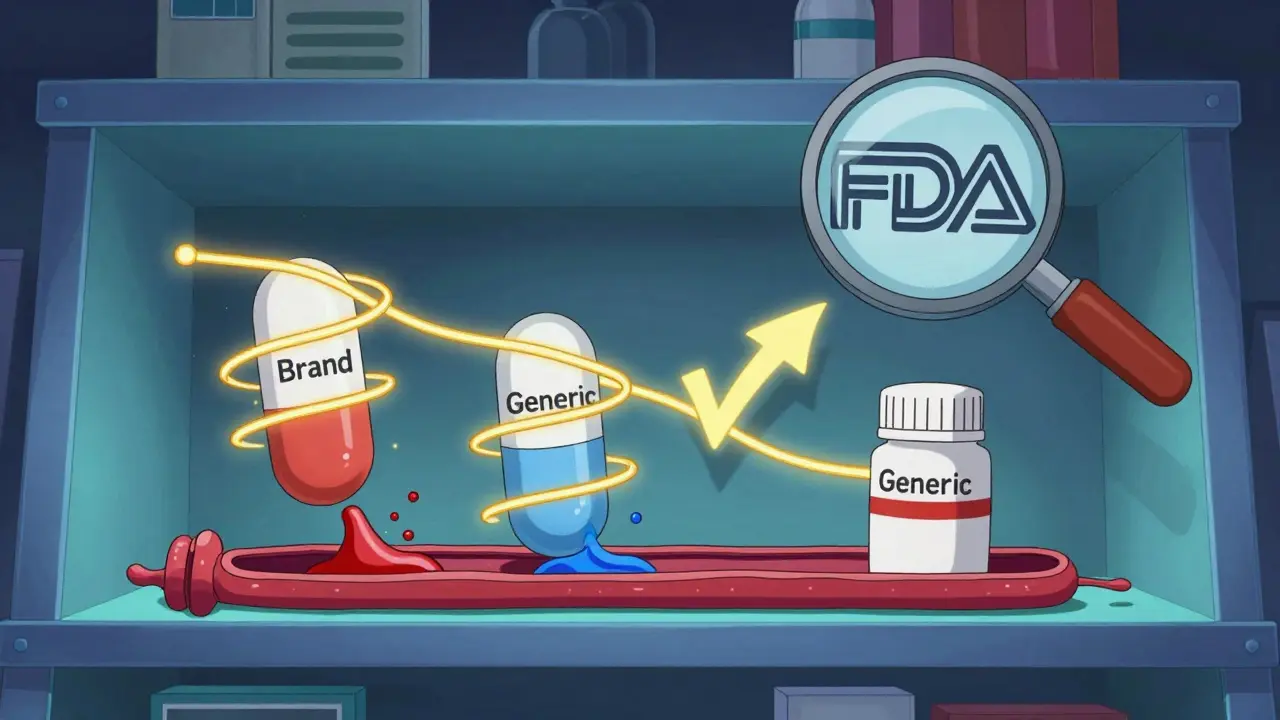
Generic Drug Approval Standards: Safety, Quality, and Strength Requirements
Generic drugs must meet the same safety, quality, and strength standards as brand-name drugs. The FDA ensures bioequivalence, strict manufacturing controls, and consistent performance through the ANDA process, making generics a reliable and cost-effective choice for millions.

Statins and Antifungal Medications: How Their Interaction Raises Rhabdomyolysis Risk
Statins and certain antifungals can dangerously interact, raising the risk of rhabdomyolysis-a severe muscle breakdown. Learn which combinations are risky, who’s most vulnerable, and how to stay safe.

Heavy Menstrual Bleeding on Blood Thinners: What Works and What to Ask Your Doctor
Heavy menstrual bleeding affects up to 70% of women on blood thinners, yet most doctors don’t screen for it. Learn safe, effective treatments-including the Mirena IUD and tranexamic acid-that work without stopping anticoagulation.

OTC Cold Medicine Safety in Children: Age Limits and Risks
OTC cold medicines pose serious risks to young children and offer little to no benefit. Learn the age limits, dangers, and safer alternatives backed by the FDA and pediatric experts.
Complex Regional Pain Syndrome: Understanding Burning Pain After Injury
CRPS causes intense burning pain after injury, often mistaken for normal healing. Learn the signs, triggers, and proven treatments to stop it before it becomes lifelong.

Dechallenge and Rechallenge in Drug Side Effects: How Doctors Confirm Which Medication Is Causing Harm
Dechallenge and rechallenge are key clinical tools used to confirm which drug is causing side effects. Stopping a medication to see if symptoms improve (dechallenge) and carefully restarting it to see if the reaction returns (rechallenge) provide the strongest evidence of drug causality in real-world practice.

FDA Therapeutic Equivalency Codes: How Laws Determine Which Generic Drugs Can Be Substituted
FDA Therapeutic Equivalency (TE) codes determine whether generic drugs can legally replace brand-name versions. Learn how A and B ratings work, why some generics can't be substituted, and how state laws and FDA science shape your prescriptions.
Parkinson’s Disease: Understanding Motor Symptoms, Medications, and Daily Living Challenges
Parkinson’s disease affects movement through tremors, stiffness, and slow motion. Medications like levodopa help, but long-term use brings side effects. Daily living requires exercise, therapy, and support to maintain independence and quality of life.

How to Bring Pill Bottles to Appointments for Accurate Medication Reconciliation
Bringing your actual pill bottles to doctor appointments is the most reliable way to prevent dangerous medication errors. Learn what to bring, why memory isn't enough, and how to prepare for accurate medication reconciliation.