When you pick up a prescription, you might assume the generic version is just as good as the brand - and you’re right. But here’s the catch: not all drugs even have a generic option, and even fewer have something called an authorized generic. And that’s not an accident. It’s by design.
What Exactly Is an Authorized Generic?
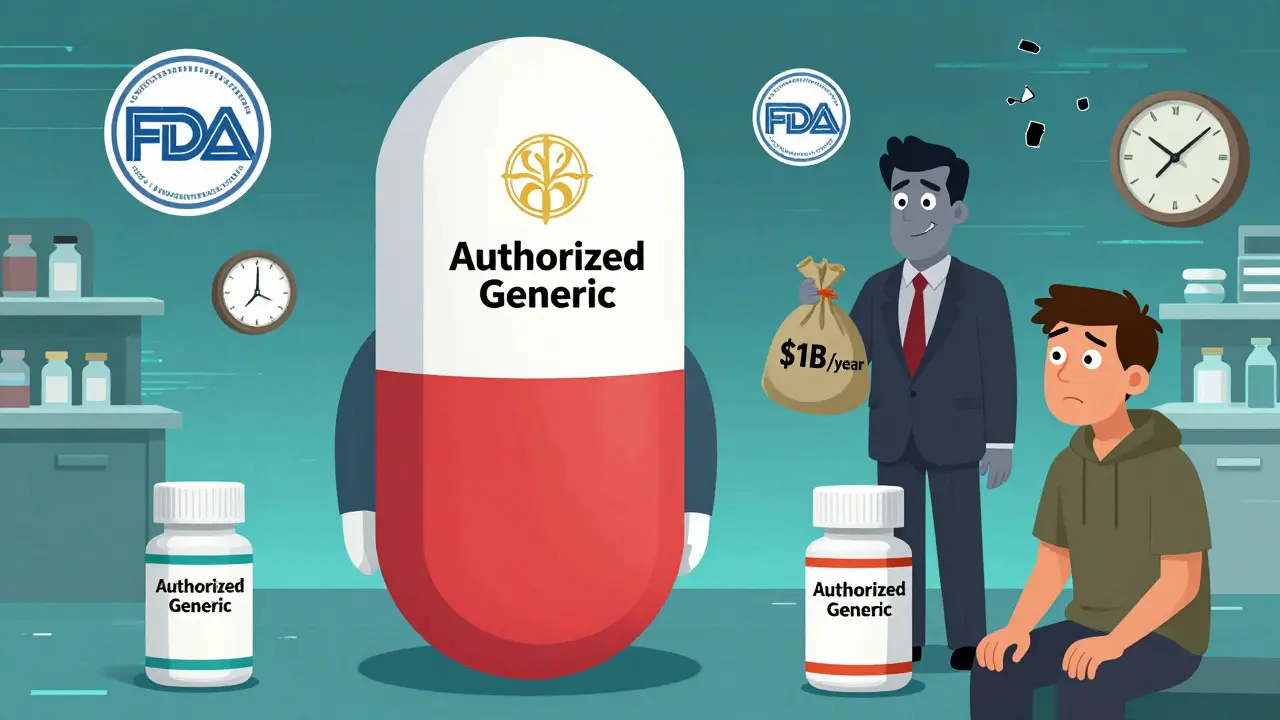
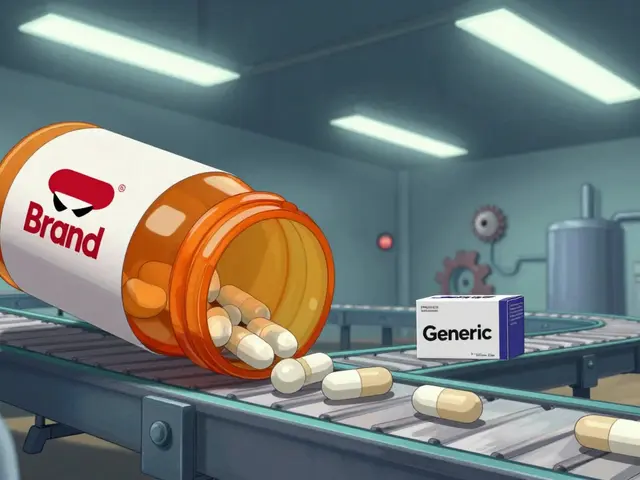
An authorized generic isn’t a copy. It’s the exact same pill, capsule, or injection as the brand-name drug - same active ingredient, same factory, same packaging, just without the brand logo. It’s made by the original drug company, under the same FDA-approved application, and sold at a lower price. Think of it like a car manufacturer selling the same model under a different badge - same engine, same seats, cheaper sticker. Unlike regular generics, which must prove they’re bioequivalent through testing, authorized generics skip that step entirely. They don’t need to show they work the same way because they are the same way. The FDA tracks them separately, and as of September 2019, there were only 1,215 authorized generics listed in the U.S. That’s out of more than 20,000 prescription drugs on the market. Most drugs you take? No authorized generic. Ever.Why Don’t All Drugs Have Authorized Generics?
The answer isn’t about science or safety. It’s about money. Brand-name drugmakers don’t launch authorized generics because they want to help patients. They do it because it helps them keep control. If a drug is making $1 billion a year and a patent is about to expire, the company has two choices: let a generic competitor take 80% of the market, or launch their own generic version first - an authorized generic - and keep most of the profits. Take Mylan’s EpiPen. In 2016, while the patent was still active, Mylan released an authorized generic priced 15% lower than the brand. Did that help patients? Sure. But it also stopped other companies from entering the market with their own generics. Why bother spending millions on a lawsuit and years of paperwork if the brand company already has a cheaper version on the shelf? This is why only 89% of top-selling drugs (those making over $1 billion a year) have used authorized generics. For drugs making less than $100 million? Only 22% ever got one. If it’s not a blockbuster, the company doesn’t bother. No profit motive. No authorized generic.The 180-Day Exclusivity Trap
Here’s where it gets complicated. When a generic company files to sell a drug after patent expiration, they get 180 days of exclusive rights - no other generic can enter during that time. It’s meant to reward them for taking the legal risk. But if the brand company launches an authorized generic during those 180 days? That exclusivity becomes worthless. The FTC found that when this happens, the first generic’s sales drop by 40% to 52%. The price of the generic plummets. The brand company wins. The generic company loses. Patients get a lower price - but only because the system was manipulated. In one case, when Teva launched an authorized generic of Protonix, the price of the brand dropped 35% almost overnight. But no other generic manufacturer dared to enter. Why risk it if the brand company can just flood the market with its own version?
Who Benefits? Who Gets Hurt?
Patients see lower prices - sometimes $18.75 less per prescription during the 180-day window. That’s real savings. But it’s temporary. Once the authorized generic is in place, there’s no pressure to drop prices further. The brand company still controls supply. Pharmacists report confusion. Walgreens staff said 27% more errors happened when both brand and authorized generic versions were available - because they look identical. Doctors are confused too. A 2018 survey found 63% of physicians struggled to know which version to prescribe. Patients get shocked when their “generic” suddenly changes packaging. They think it’s a different drug. And here’s the worst part: authorized generics discourage competition. The FTC found that when a brand company threatens to launch an authorized generic, the chance of a real generic company challenging the patent drops from 4% to 10%. That means fewer competitors over time. Fewer competitors means prices don’t keep falling.Regulators Are Watching - But Not Stopping It
The FDA started updating its authorized generic list quarterly in January 2022, after years of annual updates that left people in the dark. That’s progress. But they still don’t regulate when or why these generics are launched. The FTC has repeatedly warned Congress that authorized generics undermine the whole purpose of the Hatch-Waxman Act - which was to speed up generic competition, not block it. In 2023, they filed legal briefs asking courts to limit their use during the 180-day window. Congress has tried to act. Bills like the Preserve Access to Affordable Generics and Biosimilars Act have been reintroduced with bipartisan support. But nothing’s passed. The drug industry spends millions lobbying against restrictions.
What This Means for You
If you’re on a brand-name drug and wondering why there’s no generic, the answer might be simple: the company doesn’t want one. Or worse - they already made one, but it’s their own. Ask your pharmacist: “Is there an authorized generic for this?” If they say yes, you might save money. But if they say no, don’t assume it’s because no one made it. It’s because the company chose not to. Your best move? Compare prices. Sometimes the brand-name version is cheaper than the authorized generic because of insurance discounts. Sometimes the real generic - if it exists - is the best deal. Don’t assume. Check.What’s Next?
Biosimilars are coming. These are complex drugs - like insulin or biologics - that can’t be copied exactly. They’re the next wave of lower-cost options. But even they won’t fix the problem: drugmakers still control access. Until the law changes, authorized generics will remain a tool for brand companies to manage competition - not a tool for patients to get fair prices. They’re not evil. They’re strategic. And right now, the system rewards that strategy. The next time you see a generic on your receipt, ask yourself: Is this a real competitor? Or just the brand wearing a different hat?Are authorized generics the same as regular generics?
Yes and no. Authorized generics are made by the brand company using the exact same formula and factory as the brand-name drug. Regular generics are made by other companies and must prove they work the same way through testing. Authorized generics skip that step - they’re identical copies, just sold under a different label.
Why don’t all drugs have authorized generics?
Because drug companies only launch them when it benefits their profits. If a drug makes less than $100 million a year, there’s no financial reason to create an authorized generic. Only blockbuster drugs - those earning over $500 million - are likely to get one. It’s not about patient access. It’s about market control.
Can authorized generics lower drug prices long-term?
Usually not. They cause a short-term price drop - often during the 180-day window when the first generic is supposed to have exclusive rights. But after that, prices stabilize. Because the brand company controls the authorized generic, they can limit supply or raise prices later. Real competition from independent generic makers rarely follows.
Do authorized generics confuse patients and pharmacists?
Yes. Many patients don’t realize their “generic” is actually made by the same company as the brand. Pharmacists report more errors when both versions are available because the pills look identical. Some patients get upset when their medication suddenly changes packaging - thinking it’s a different drug.
Is there a law that stops companies from using authorized generics to block competition?
Not yet. The FTC and Congress have raised concerns, and bills have been introduced to ban authorized generics during the 180-day exclusivity period. But no federal law currently prevents it. The system still allows brand companies to use authorized generics as a legal way to delay real generic competition.







Kelly Beck
January 7, 2026 AT 00:05Okay but can we just take a second to appreciate how wild it is that the same pill you pay $200 for comes out of the exact same machine as the one costing $15? Like, imagine if your iPhone was made in the same factory as the $50 knockoff but they charged you extra for the logo. It’s not capitalism, it’s performance art. 🤯
Beth Templeton
January 7, 2026 AT 05:08Authorized generics are just brand companies playing chess while patients think they’re playing checkers.
Wesley Pereira
January 8, 2026 AT 05:11Bro the 180-day exclusivity loophole is pure corporate jujitsu. You file for a generic, they drop their own version on day one, and suddenly you’re not a disruptor-you’re a footnote. FDA’s quarterly list update? Cute. They’re not regulating the game, they’re just updating the scoreboard. Meanwhile, my insulin still costs more than my rent.
Katie Schoen
January 10, 2026 AT 05:03Also real quick-has anyone else noticed that when your 'generic' changes packaging mid-script, your pharmacist just shrugs like it’s normal? Like we’re all just supposed to accept that our anxiety meds now look like a candy from 2003? No one asks why. No one questions it. We just pop it and hope it still works. 🤷♀️
Melanie Clark
January 10, 2026 AT 18:34Let me guess the FDA is too busy writing quarterly reports to actually stop this scam. This isn’t a market failure this is a coordinated oligopoly with a side of bureaucratic negligence. They’re not just selling drugs they’re selling illusions. The brand name is the placebo and the authorized generic is the truth they don’t want you to see. I swear if I find out my blood pressure pill was made by Pfizer all along I’m going to scream into a pillow for an hour
Dana Termini
January 11, 2026 AT 07:32I get why companies do it but it still feels wrong. I’ve had patients cry because they thought their new 'generic' was a different drug. It’s not just about cost-it’s about trust. And when your pharmacy gives you a different-looking pill every month, that trust evaporates. We need better labeling. Not more loopholes.
Ryan Barr
January 12, 2026 AT 13:40The fact that you think this is surprising reveals your lack of understanding of pharmaceutical economics. It’s not malice-it’s rational profit maximization within a flawed regulatory framework. You don’t like it? Advocate for structural reform, not emotional outrage.
Molly McLane
January 14, 2026 AT 12:18Hey everyone-just wanted to say I’m so glad this thread is happening. We’re finally talking about how the system is rigged against patients, and that’s huge. I work in community health and I see this every day. People skip doses because they can’t afford the brand, then panic when the generic looks different. We need transparency. We need labeling that says ‘Made by [Brand Name]’ on authorized generics. Simple. Human. Maybe then we can start rebuilding trust. And if Congress won’t act? We vote with our wallets and our voices. You’re not alone in this.
Tiffany Adjei - Opong
January 14, 2026 AT 21:02Wait so you’re saying the system isn’t broken-it’s working exactly as intended? Then why are we pretending this is a healthcare issue and not a corporate power play? Also I read that the same people who lobby against generic reform also donate to both parties. So yeah. We’re not just being screwed. We’re being sponsored.