Tag: bioequivalence

How the FDA Ensures Generic Drugs Work the Same as Brands
The FDA ensures generic drugs work the same as brand-name drugs through strict bioequivalence testing, identical active ingredients, and rigorous manufacturing standards. Over 90% of U.S. prescriptions use generics, saving billions annually.
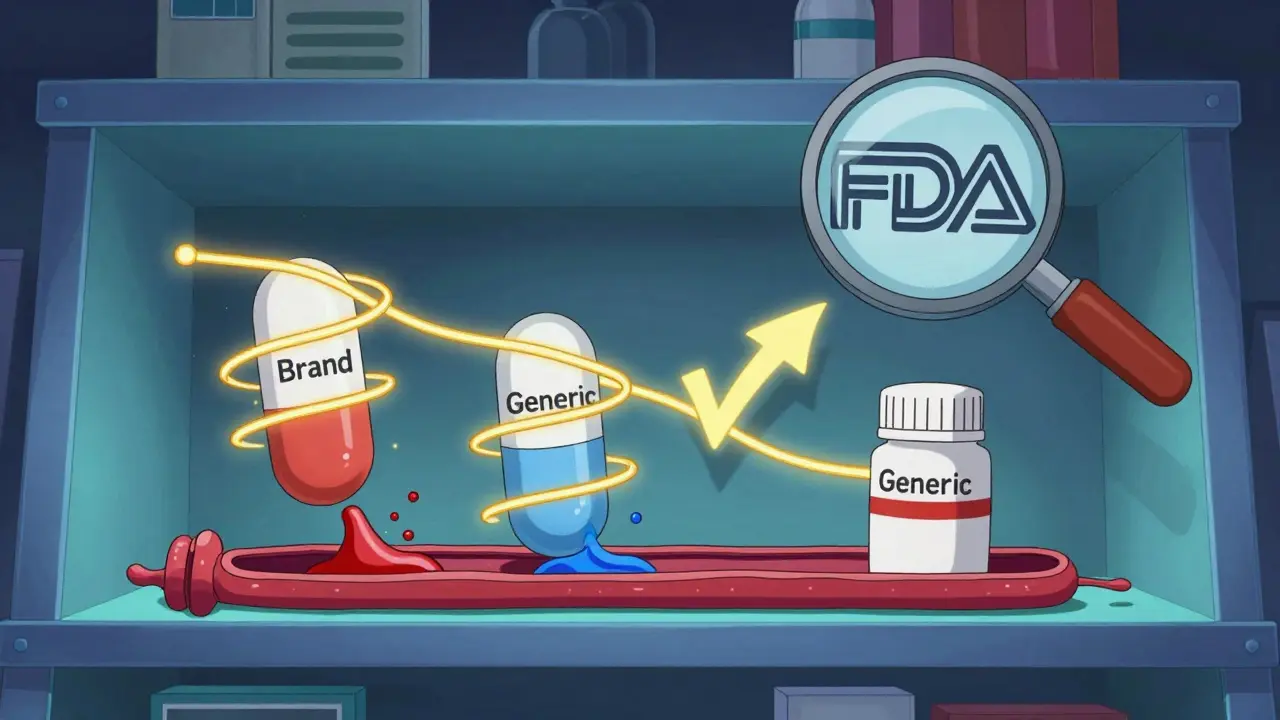
Generic Drug Approval Standards: Safety, Quality, and Strength Requirements
Generic drugs must meet the same safety, quality, and strength standards as brand-name drugs. The FDA ensures bioequivalence, strict manufacturing controls, and consistent performance through the ANDA process, making generics a reliable and cost-effective choice for millions.

ANDA Process: Legal Requirements for Generic Drug Approval in the U.S.
The ANDA process is the legal pathway for generic drug approval in the U.S., requiring bioequivalence, identical active ingredients, and strict manufacturing standards under the Hatch-Waxman Act. Learn the key requirements, costs, and pitfalls.