Tag: generic drug quality
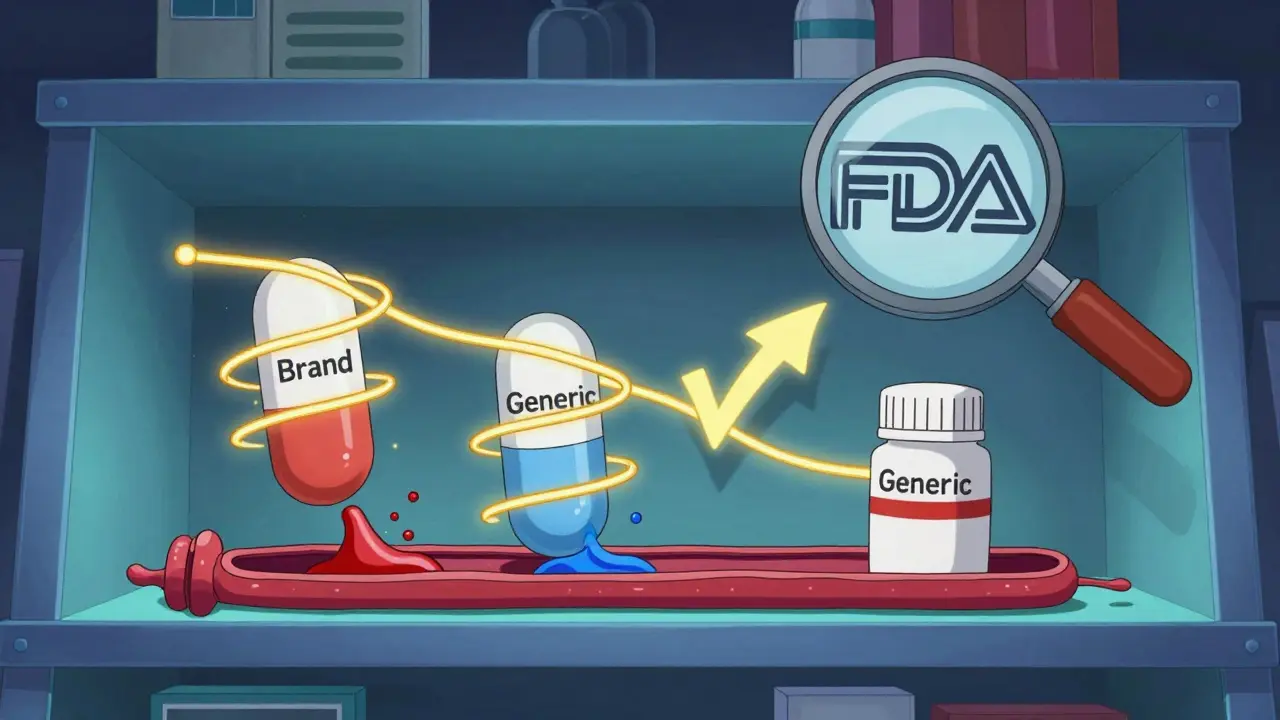
Generic Drug Approval Standards: Safety, Quality, and Strength Requirements
Generic drugs must meet the same safety, quality, and strength standards as brand-name drugs. The FDA ensures bioequivalence, strict manufacturing controls, and consistent performance through the ANDA process, making generics a reliable and cost-effective choice for millions.

GMP for Generics: FDA Requirements for Manufacturing
Learn the FDA's real CGMP requirements for generic drug manufacturing-what’s mandatory, what fails most often, and how companies stay compliant. No fluff, just the facts that keep millions of pills safe.




