Generic Substitution: What It Is, When It Works, and When to Avoid It
When your pharmacist hands you a pill that looks different from what you’re used to, it’s likely generic substitution, the practice of replacing a brand-name drug with a chemically identical version approved by the FDA. Also known as generic switching, it’s meant to cut costs without sacrificing effectiveness—but it doesn’t always work the way you think. Not all generics are created equal. Some are exact copies of the brand, down to the inactive ingredients. Others? They’re close enough to pass FDA tests but might behave differently in your body—especially if you’re on narrow-therapeutic-index drugs like warfarin, levothyroxine, or seizure meds.
Then there’s authorized generics, the same drug made by the original brand company but sold without the brand name or fancy packaging. Also known as same-drug generics, they’re often cheaper than the brand and identical in every way—including how your body absorbs them. Unlike traditional generics, which are made by different manufacturers, authorized generics don’t have the same variability risks. Meanwhile, traditional generics, lower-cost versions produced by third-party companies after patents expire, rely on bioequivalence studies that allow for up to a 20% difference in absorption rate. For most people, that’s fine. For others, it’s the difference between control and crisis.
Why does this matter? Because generic substitution isn’t just about price. It’s about consistency. If you’re on a medication where tiny changes in dosage can trigger side effects—like thyroid meds, blood thinners, or epilepsy drugs—switching generics without your doctor’s okay can be risky. Some patients report headaches, mood swings, or even seizures after a switch, even though the pills are "the same." That’s not a myth. It’s real. And it’s why some doctors and pharmacists recommend sticking with the same generic manufacturer—or better yet, asking for an authorized generic if cost is an issue.
Not every drug needs to stay brand-name. For antibiotics, antacids, or pain relievers, generic substitution works just fine. But for chronic conditions where stability matters, knowing the difference between a generic and an authorized generic can save you from unnecessary health bumps. You don’t need to pay more. You just need to know what you’re getting. Below, you’ll find real stories and practical guides from people who’ve been there—how to spot the right generic, how to talk to your pharmacist about switching, and when it’s smarter to stick with what you know.
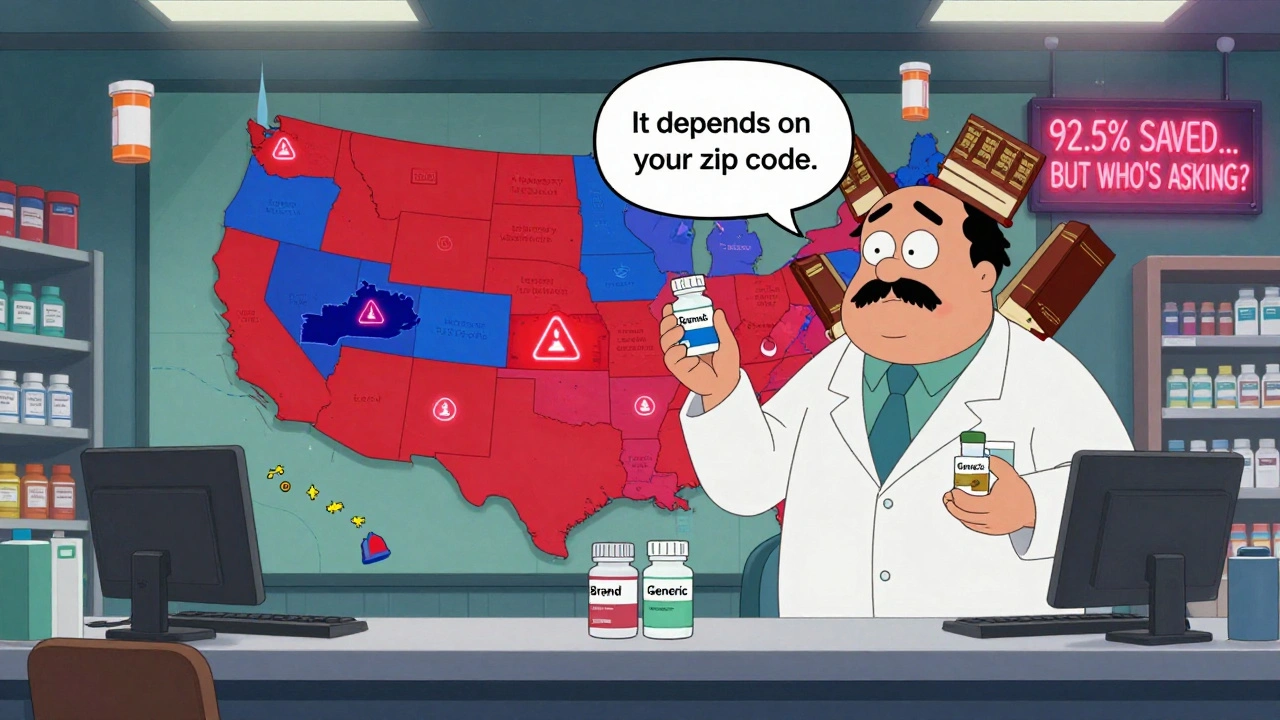
State Laws on Generic Drug Substitution: How Rules Vary Across the U.S.
State laws on generic drug substitution vary widely across the U.S., affecting how pharmacists replace brand-name drugs with cheaper generics. These rules impact cost, safety, and patient trust-especially for critical medications.

Medication Therapy Management: How Pharmacists Optimize Generic Drug Use for Better Outcomes
Pharmacists play a vital role in Medication Therapy Management by optimizing generic drug use to improve adherence, reduce costs, and prevent adverse events. Learn how MTM works and why it matters for patients on multiple medications.