Tag: ANDA
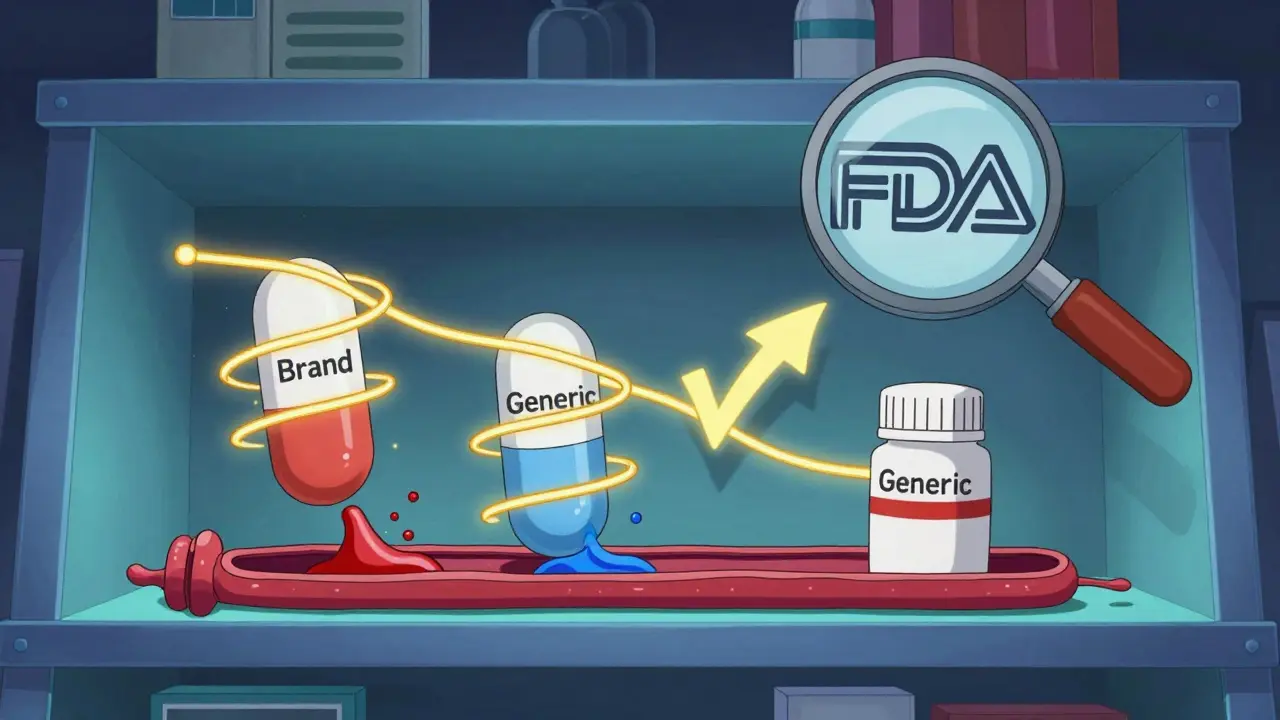
Generic Drug Approval Standards: Safety, Quality, and Strength Requirements
Generic drugs must meet the same safety, quality, and strength standards as brand-name drugs. The FDA ensures bioequivalence, strict manufacturing controls, and consistent performance through the ANDA process, making generics a reliable and cost-effective choice for millions.