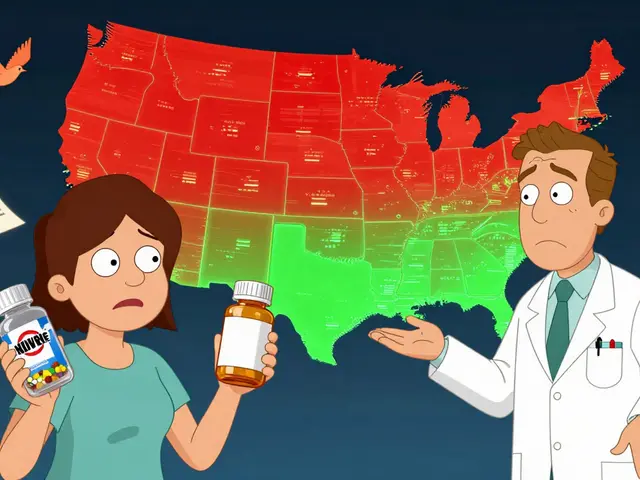
Every year, Americans fill over 4 billion prescriptions. About 9 out of 10 of those are for generic drugs. But here’s the thing: just because a generic is available doesn’t mean you’ll automatically get it. That decision isn’t made by your doctor, your pharmacist, or even your insurance company alone. It’s ruled by the state you live in.
Each of the 50 states and Washington, D.C. has its own rules about when and how a pharmacist can swap a brand-name drug for a cheaper generic version. Some states say pharmacists must substitute. Others say they can-but only if you say yes. And in a few, certain drugs like epilepsy meds or blood thinners are off-limits for substitution, no matter what.
These rules aren’t just paperwork. They affect how much you pay, whether your medication works the same way, and even whether your pharmacist can legally switch your prescription without asking. If you’ve ever been handed a different-looking pill bottle and wondered why, this is why.
What Exactly Is Generic Substitution?
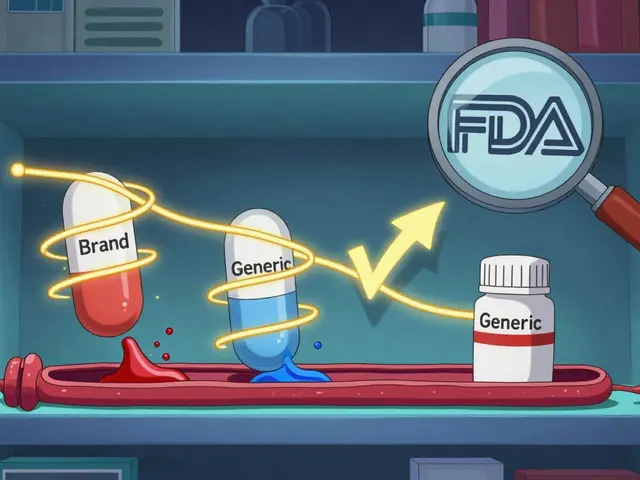
Generic substitution means replacing a brand-name drug with a chemically identical version that costs less. The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand. They must also meet the same quality and safety standards.
The key term here is therapeutic equivalence. The FDA tracks this in the Orange Book, a public database that rates drugs as either “AB” rated (interchangeable) or not. If a generic is AB-rated, it’s legally and clinically considered a direct replacement. But that doesn’t mean you’ll automatically get it.
State laws control whether substitution happens automatically, only with permission, or never at all. That’s where things get messy.
Mandatory vs. Permissive Substitution Laws
There are two main types of state laws: mandatory and permissive.
In 19 states-including California, New York, and Texas-pharmacists are required to substitute a generic when it’s available, unless the prescriber writes “dispense as written” or “do not substitute.” This is called a mandatory substitution law. These states assume that if a generic is approved by the FDA, it’s safe to use. The goal? Cut costs across the board.
The other 31 states and D.C. use permissive substitution. That means pharmacists can switch to a generic, but they don’t have to. In these places, the decision often depends on whether the patient agrees. Some of these states, like Alaska and Maine, require pharmacies to post signs saying substitutions are possible. Others don’t even do that.
Here’s the kicker: states with mandatory laws see generic use rates 8 to 12 percentage points higher than permissive states. That translates to $50 to $150 saved per prescription, on average. But not every state wants to push that hard.
When Do You Need to Say Yes?
Seven states-Connecticut, Hawaii, Maine, Maryland, New Hampshire, Vermont, and West Virginia-plus Washington, D.C., require you to give explicit consent before a substitution can happen. That means your pharmacist has to ask you, face to face or in writing, and you have to say “yes.”
Why? Because some drugs have a narrow therapeutic index. That means tiny changes in dose or absorption can cause big problems. Think warfarin (a blood thinner), levothyroxine (for thyroid), or anti-seizure meds like phenytoin. Even small differences between brands and generics can trigger seizures, bleeding, or other dangerous side effects.
Hawaii takes it further: it bans substitution of anti-seizure drugs unless both the doctor and the patient give written consent. Kentucky does something similar for a short list of high-risk drugs.
Even in states that don’t require consent, 31 states and D.C. still require pharmacists to notify you after the switch. That means your receipt, your prescription label, or a handout might say “Generic dispensed instead of brand.” But if you don’t read it? You might never know.

What About Biosimilars?
Biosimilars are the generic version of biologic drugs-complex medications made from living cells, like Humira or Enbrel. They’re not as simple to copy as pills. The FDA has a special “interchangeable” designation for biosimilars, meaning they can be substituted without the prescriber’s involvement.
But here’s the twist: 45 states have stricter rules for biosimilars than for regular generics. Six states that require generic substitution still treat biosimilars as optional. That means if you’re on a biologic, your pharmacist might not be allowed to switch you-even if the FDA says the biosimilar is interchangeable.
And 48 states plus D.C. require pharmacists to tell your doctor within 2 to 7 days after switching you to a biosimilar. If your doctor doesn’t get that notice, they might think your medication stopped working-or worse, they might refill the wrong one.
Who’s Liable If Something Goes Wrong?
Pharmacists are on the front lines. They’re the ones filling the script, checking the label, and making the call. But if a substituted drug causes harm, who’s responsible?
Twenty-four states-including Alabama, Illinois, Missouri, and Oregon-don’t give pharmacists any legal protection from liability if a substitution leads to an adverse reaction. That means if you have a bad reaction after a switch, your pharmacist could be sued-even if they followed state law.
That’s why some pharmacists avoid substitutions in those states. Especially with high-risk drugs. One pharmacist in Ohio told a reporter: “I’d rather get yelled at for not switching than risk a lawsuit.”
Meanwhile, states like Arizona and California have clear liability protections built into their laws. That gives pharmacists more confidence to make substitutions without fear.
How Pharmacists Keep Up With 51 Different Rules
Imagine you work at a pharmacy chain that spans three states. One day you’re filling a script in Pennsylvania. The next, you’re in New Jersey. Then you’re in Maryland. Each state has different rules about consent, notification, and which drugs can’t be switched.
It’s not just confusing-it’s time-consuming. A 2021 survey found that 68% of independent pharmacists spend 15 to 30 minutes a day just managing substitution rules. That’s time that could be spent counseling patients.
Big pharmacy chains like Walgreens and CVS have built software that auto-detects the state and applies the right rules. Epic’s “State Substitution Rules Engine,” launched in 2019, cuts substitution errors by 37%. But smaller pharmacies? Many still rely on printed handbooks or online databases like the National Association of Boards of Pharmacy’s interactive map.
The Accreditation Council for Pharmacy Education requires pharmacists to take 2 hours of continuing education on substitution laws every two years. But pharmacists who live near state borders say they spend 8 to 12 extra hours a year just staying current.

What’s Changing in 2025?
The system is starting to crack under its own weight. In 2023, 12 states introduced bills to standardize substitution laws. The goal? Make rules consistent so pharmacists don’t have to be legal experts.
The FDA updated its Orange Book in 2022 to include new “interchangeability” ratings for complex generics-like inhalers or injectables. That triggered 18 states to review their laws. And in 2023, a study in Health Affairs showed that states that simplified their rules saw generic use jump by nearly 7% in just a few years.
Meanwhile, biosimilar adoption is still slow. Only 11% of biologic prescriptions are filled with biosimilars. That’s partly because patients don’t know they’ve been switched. A 2022 survey by the National Psoriasis Foundation found that 42% of patients had no idea their biologic had been changed to a biosimilar-even though their state required notification.
And the cost of not standardizing? The Congressional Budget Office estimates that by 2030, inconsistent laws will cost the system $4.7 billion a year in avoidable brand-name drug use.
What You Should Do
If you’re on a generic medication and want to make sure you’re getting the right one:
- Check your prescription label. Does it say “generic” or the brand name?
- Ask your pharmacist: “Was this switched from the brand?”
- If you’re on a high-risk drug (blood thinners, thyroid meds, seizure meds), ask if substitution is allowed in your state.
- Know your state’s rules. If you live in Connecticut or Hawaii, you have a right to say no.
- Keep a list of your meds and what form you take. If your pill looks different, don’t assume it’s the same.
Most people never think about this until something goes wrong. But with so many rules, so many drugs, and so many states, it’s not enough to trust the system. You have to ask.
Why This Matters
Generic drugs save billions every year. They’re safe. They’re effective. But the patchwork of state laws makes it harder than it should be.
Some states want to save money. Others want to protect patients. Both are valid. But when the rules change every time you cross a border, the system fails everyone: patients, pharmacists, and doctors.
The future of drug access isn’t just about new medicines. It’s about making sure the ones we already have-cheaper, proven, safe ones-can actually reach the people who need them. And that starts with fixing the rules.
Can my pharmacist switch my brand-name drug without telling me?
In 31 states and Washington, D.C., pharmacists can switch your brand-name drug to a generic without asking your permission-but they must notify you after the fact, usually on your receipt or label. In 7 states plus D.C., they must get your explicit consent before switching. Always check your prescription label and ask if you’re unsure.
Are all generic drugs safe to substitute?
The FDA approves generics as therapeutically equivalent to brand-name drugs, meaning they work the same way. But for drugs with a narrow therapeutic index-like warfarin, levothyroxine, or certain anti-seizure medications-even small differences can be risky. Some states ban substitution for these drugs entirely. Always confirm with your pharmacist if your medication falls into this category.
Why do some states require patient consent and others don’t?
States with consent requirements are usually responding to concerns about patient safety, especially with high-risk medications. These laws were often pushed by medical groups like the American Epilepsy Society. States without consent laws prioritize cost savings and efficiency, assuming FDA-approved generics are interchangeable. The debate centers on balancing safety with affordability.
Can I request a brand-name drug even if a generic is available?
Yes. In every state, you can ask your pharmacist to dispense the brand-name drug instead of the generic. You may have to pay more out-of-pocket, especially if your insurance doesn’t cover the brand. Your prescriber can also write “dispense as written” on the prescription to prevent substitution.
How do I find out what my state’s substitution laws are?
The National Association of Boards of Pharmacy (NABP) offers a free, interactive map on their website that shows current substitution laws for every state. Your pharmacist should also be able to tell you your state’s rules. If you’re unsure, ask: “What are the rules here for switching my meds?”
Do these laws apply to biosimilars too?
Yes, but differently. All states require biosimilars to be FDA-designated as “interchangeable” before substitution. But 45 states have stricter rules for biosimilars than for regular generics. Many require pharmacist notification to the prescriber and some prohibit substitution without patient consent-even if the drug is interchangeable. Always confirm if your biologic was switched to a biosimilar.
What happens if I get the wrong drug because of a substitution error?
If a substitution error causes harm, liability depends on your state’s laws. In states with no pharmacist liability protection, the pharmacist or pharmacy may be held responsible. In others, the law shields them if they followed state rules. Always report any unexpected changes in your medication to your doctor and pharmacist immediately.





Meghan Hammack
January 9, 2026 AT 05:56Just wanted to say - if you’re on thyroid meds or blood thinners, ALWAYS ask your pharmacist if it was switched. I learned the hard way when my levothyroxine dose felt off and I didn’t realize they’d swapped brands. Took me three weeks to figure it out. Don’t be like me. Ask. Every. Time. 💪
Gregory Clayton
January 9, 2026 AT 16:44Ugh, another ‘state-by-state’ rant. We’re one country, not 51 different nations with different rules about pills. Just make it federal already. Why does my pharmacy in Ohio need a law degree to fill a script? 🤦♂️
Jeffrey Hu
January 11, 2026 AT 07:42Actually, the FDA’s Orange Book doesn’t just rate drugs as ‘AB’ - it has subcategories like AB1, AB2, etc., based on bioequivalence studies. Most people don’t realize that even ‘interchangeable’ generics can have different inactive ingredients, which can affect absorption in sensitive patients. It’s not just about active ingredients - excipients matter too. And no, your pharmacist isn’t trained to explain that. 😅
tali murah
January 12, 2026 AT 12:50Oh wow. So pharmacists in 24 states are legally vulnerable for doing their job correctly? And we wonder why they’re burnt out? The system isn’t broken - it was designed to fail. Let’s just make the FDA the sole authority and cut the state bureaucracy. Or better yet - let insurance companies handle it. They’re already the real drug gatekeepers anyway. 🙄
Jerian Lewis
January 13, 2026 AT 00:08People think generics are ‘just as good.’ They’re not. They’re chemically identical, yes - but manufacturing quality varies wildly. I’ve seen generics from the same company that look different, taste different, even cause different side effects. It’s not paranoia. It’s science. And no, the FDA doesn’t monitor every batch. Stop trusting the system.
Alicia Hasö
January 14, 2026 AT 10:41To everyone reading this: you have power. You can say no. You can ask for the brand. You can demand to know if your medication changed. You’re not just a patient - you’re the most important part of this system. Don’t let fear or apathy silence you. Your health isn’t a cost-cutting experiment. Speak up. Write it down. Ask again. You’ve got this. 💛
Maggie Noe
January 15, 2026 AT 10:47Just had my biosimilar swapped last month. Didn’t know until I saw the label. Now I’m having weird joint pain. I asked my doc - he said ‘it’s fine, FDA-approved.’ But I don’t feel fine. 🤕 Why does no one tell you this stuff BEFORE it happens? I feel like a lab rat. 🐀
Pooja Kumari
January 17, 2026 AT 06:31OMG I’m from India and I can’t believe how complicated this is here. In my country, generics are everywhere and no one asks - it’s just cheaper, so you take it. But here? You need a lawyer, a consent form, a notary, and a signed affidavit just to get a pill? And then you still don’t know if it’s the same? I’m crying. Why is America so obsessed with paperwork? It’s a pill, not a passport. 😭
Catherine Scutt
January 18, 2026 AT 20:57Wow. So the system is designed to confuse people so pharmacists can avoid liability? Brilliant. Just brilliant. No wonder people stop taking meds. You’re supposed to be the expert, but you’re too scared to do your job? And the patient pays the price. Classic.
Aron Veldhuizen
January 20, 2026 AT 12:16You all miss the point. This isn’t about generics. It’s about control. Who gets to decide what you take? The FDA? The state? The pharmacist? The insurance company? Or you? The real question is: why does any of this matter more than your right to autonomy? You’re being manipulated by bureaucracy disguised as safety. The pill doesn’t care about your state line. Neither should you.