Degradation in Pharmaceuticals: How Drugs Break Down and Why It Matters
When we talk about degradation, the chemical breakdown of a drug over time that reduces its potency or creates harmful byproducts. Also known as drug instability, it’s not just a lab concern—it’s what happens in your medicine cabinet if you leave pills in a hot bathroom or past their expiration date. This isn’t theoretical. A study from the FDA found that some antibiotics lose over 90% of their strength after just 6 months if stored improperly. That means your doxycycline or ciprofloxacin might not work at all—no matter how fresh the prescription seems.
Drug stability, how well a medication holds up under heat, light, moisture, and time. Also known as pharmaceutical stability, it’s the reason manufacturers run stability testing for years before a drug even hits shelves. These tests follow strict ICH guidelines to predict shelf life and prevent recalls. But it’s not just about the factory. Your home environment matters too. Antacids like Tums can speed up degradation in certain antibiotics if taken together, and dairy products can interfere with absorption—both are forms of chemical interference that mimic degradation effects. Even something as simple as leaving your insulin in a hot car can cause it to degrade, making it useless or even dangerous.
Shelf life, the period during which a drug remains safe and effective under recommended storage conditions. Also known as expiration date, it’s not a guess—it’s based on real-world degradation data. That date on your bottle isn’t arbitrary. It’s the result of controlled studies tracking how the active ingredient breaks down over time. Some drugs, like nitroglycerin, degrade within months. Others, like tablets, can last years if kept dry and cool. But once you open a bottle or break the seal, the clock starts ticking faster. Moisture, air, and temperature swings are the enemies. That’s why emergency go-bags for meds include cool packs and sealed containers. It’s not overkill—it’s science.
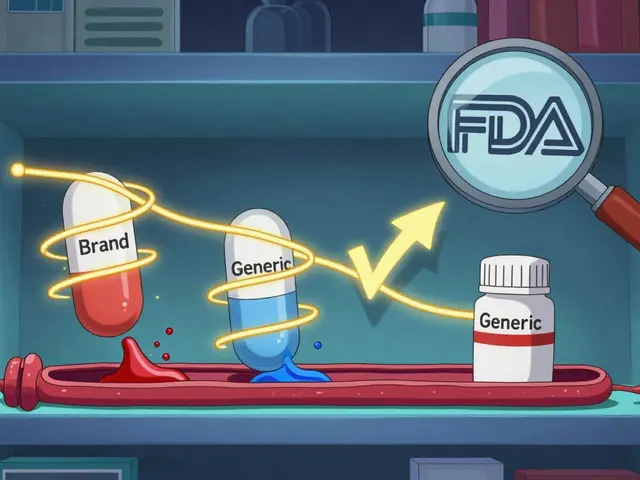
Understanding degradation helps you avoid treatment failure. If your seizure medication loses potency because you stored it in a sunny window, your seizures could return. If your antihypertensive breaks down in humidity, your blood pressure could spike. And if you’re using generic drugs without knowing whether they’re authorized generics (same formula, no brand label), you’re trusting the same stability standards—but you should verify.
What you’ll find below are real, practical stories from people who’ve been affected by degradation—whether it’s a failed antibiotic course because they took it with milk, a diabetic who lost insulin to heat, or someone who didn’t realize their antacids were neutralizing their meds. These aren’t hypotheticals. They’re cases that show why degradation isn’t just a lab term—it’s a daily health risk you can control.

Stability and Shelf Life: How Generic Products Degrade and Why Safety Matters
Stability and shelf life determine whether generic medications remain safe and effective over time. Learn how degradation happens, why storage matters, and what you need to know to protect your health.