Oct 24, 2009
Iressa (gefitinib) Improves Progression-free Survival over Standard Chemotherapy
Iressa (gefitinib) Improves Progression-free Survival over Standard Chemotherapy in Patients with NSCLC with EGFR Mutations.
Researchers from Japan have reported that Iressa (gefitinib) alone improves outcomes of patients with non–small cell lung cancer (NSCLC) with epithelial growth factor receptor (EGFR) mutations compared with patients receiving Paraplatin (carboplatin) and Taxol (paclitaxel). The details of this study were presented at the Joint ECCO 15 – 34th ESMO Multidisciplinary Congress in Berlin, September 20-24, 2009.
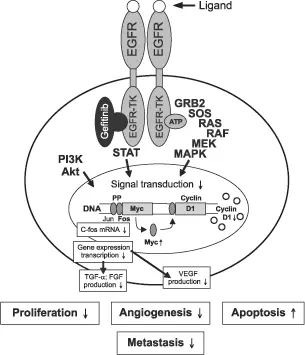
Oral Iressa (gefitinib) is approved in the United States as a single-agent treatment for patients with advanced NSCLC who have failed platinum- and taxane-based treatment. Iressa is a selective inhibitor of EGFR-tyrosine kinase. Epidermal growth factor receptor is expressed, overexpressed, or dysregulated in many human solid tumors, including NSCLC. Activation of this receptor is believed to promote tumor growth by blocking apoptosis and by increasing cell proliferation, adhesion and invasive capacity, and motility. Responsive lung tumors are likely to be adenocarcinomas or bronchio-alveolar carcinomas and occur more frequently in non-smokers and women. Responses also occur more frequently in patients with specific mutations of EGFR. Recently, researchers from Asia have reported that first-line treatment with Iressa improves progression-free survival (PFS) over combination treatment with Paraplatin and Taxol in advanced NSCLC among nonsmokers and former light smokers.
The current study included 198 patients with NSCLC with sensitive EGFR mutations who had received no prior chemotherapy. They were randomly allocated to receive Iressa alone or Paraplatin and Taxol.
Overall response rate was 74.5% for patients receiving Iressa versus 29% for patients receiving Paraplatin and Taxol. Grade 4 neutropenia occurred in 1% of patients receiving Iressa and 33% of patients receiving chemotherapy. Grade 3-4 liver dysfunction occurred in 33% of patients receiving Iressa versus 1% receiving chemotherapy. Grade 3 neuropathy occurred in 0% of patients receiving Iressa and 5% of patients receiving chemotherapy. PFS was 10.4 months following Iressa treatment versus 5.5 months for chemotherapy. Overall survival was 28.0 months for patients receiving Iressa and 23.6 months for patients receiving chemotherapy (P=0.357).
This study shows that Iressa improves PFS in patients with NSCLC with EGFR mutations compared with conventional chemotherapy. These authors suggest that Iressa be considered the new standard treatment for “sensitive EGFR mutation-positive NSCLC patients.”