Oct 27, 2009
Bortezomib in multiple myeloma
“This novel proteasome inhibitor appears to improve response rates and
survival in patients with progressive multiple myeloma.”
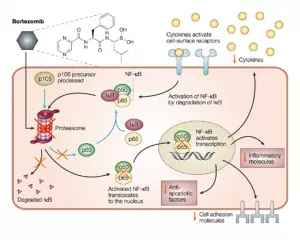
Bortezomib (Velcade) is an inhibitor of the proteasome, a ubiquitous multi-enzyme complex that degrades proteins that regulate cell-cycle progression and induces proteolysis of IκB, the inhibitor of nuclear factor-κB. Increased activation of nuclear factor-κB promotes cell survival, stimulates growth, and inhibits apoptosis, as well as induces drug resistance in myeloma cells. Recent studies have demonstrated the efficacy and safety of bortezomib in patients with relapsed, refractory myeloma.
Efficacy studies
In the phase II SUMMIT study (n = 202), treatment with bortezomib 1.3 mg/m2 twice weekly for 2 weeks, followed by 1 week without treatment, for up to 8 cycles (24 weeks) resulted in an overall response rate of 35% (including a complete response in 4% in whom myeloma protein was undetectable and a complete response in 6% in whom myeloma protein was detectable only by immunofixation).1 In the phase II CREST study, treatment with bortezomib 1.0 mg/m2 (n = 28) or 1.3 mg/m2 (n = 26) on days 1, 4, 8, and 11 every 3 weeks for up to 8 cycles (6 months) resulted in an overall response rate of 33% (4% complete response) for the 1.0-mg/m2 dose and 50% (4% complete response) for the 1.3-mg/m2 dose.2 A group of 14 patients from the CREST study and 43 from the SUMMIT study who had a partial or minimal response to bortezomib or stable disease continued receiving the proteasome inhibitor in an extension study. Most of these patients had already received 8 cycles of bortezomib therapy in the original studies. Preliminary data from the extension study indicated that bortezomib can be administered for up to 13 cycles with a safety profile similar to that observed in the first 8 cycles of treatment.
Safety demonstrated in renally impaired patients
Renal impairment is a common complication of myeloma and its treatment, and preclinical studies indicate that bortezomib and its metabolites are eliminated by both the renal and hepatic route. An analysis of outcomes in 10 patients with severe renal impairment (creatinine clearance, 10–30 mL/min) in these two studies suggests that bortezomib may be safely given to such patients, with responses and toxicities being comparable to those in patients without severe renal impairment.3 Of the 10 patients, 2 had a partial response and 1 had a minimal response; treatment was well tolerated, with 7 patients receiving at least 30 of the 32 possible study doses.
Pharmacokinetic analysis of eight patients with an initial creatinine clearance rate of 31–169 mL/min in the SUMMIT study indicated that the maximum concentration and distribution half-life of bortezomib were not affected by renal status and that the area under the concentration-time curve was similar to that obtained in the overall population.
Superior to dexamethasone
The APEX Study Group recently reported findings from their international, multicenter phase III trial comparing bortezomib with dexamethasone in patients with relapsed multiple myeloma.4 Patients were randomized to receive intravenous bortezomib 1.3 mg/m2 (n = 327) on days 1, 4, 8, and 11 every 3 weeks for 8 cycles, followed by the same dose on days 1, 8, 15, and 22 every 5 weeks for 3 cycles, or oral dexamethasone 40mg (n = 330) on days 1–4, 9–12, and 17–20 every 5 weeks for 4 cycles, followed by 40 mg on days 1–4 every 4 weeks for 3 cycles. At the time of the interim analysis, 254 progressive disease events had occurred. Median time to disease progression (using European Group for Blood and Marrow Transplantation criteria) was 5.7 months in the bortezomib-treated group versus 3.6 months in the dexamethasone-treated group (P
Significantly fewer patients taking bortezomib than those who were treated with dexamethasone developed grade 3 or worse infections (6.7% vs 10.6%, P = 0.096). No other major differences in safety were observed between treatment groups, and no difference was observed between treatment groups with regard to time to skeletal events On the basis of the interim analysis, it was recommended that the dexamethasone treatment arm be terminated in the APEX Study, and patients in the dexamethasone treatment group were permitted to receive bortezomib.
The major side effects of bortezomib are gastrointestinal symptoms, transient thrombocytopenia, fatigue, and peripheral neuropathy. Other, less frequent side effects include fever, rash, headache, and dizziness.
